Professor Dr. Wolfgang Stein
Alfried Krupp Senior Fellow
(Oktober 2021 - September 2022)
- Studium der Biologie an der Universität Kaiserslautern
Wissenschaftlicher Mitarbeiter an der Universität Bielefeld und an der Universität Ulm
- Professor für Neurophysiology, School of Biological Sciences, Illinois State University, Normal, USA
Fellow-Projekt: Die Neurobiologie der Meereserwärmung
Der andauernde Klimawandel hat zu einer beispiellosen Erwärmung der Ozeane und einer Zunahme von Wetterextremen geführt. Dies hat massive Auswirkungen auf marine Lebewesen, wie z.B. dekapode Krebse, die in besonders betroffenen Habitaten leben. Unser Verständnis darüber, wie das Nervensystem, welches das Verhalten und Überleben dieser Tiere bestimmt, auf klimawandel-bedingte Temperaturschwankungen reagiert, ist sehr limitiert. Mein Projekt charakterisiert, wie flexibel Krebse und ihr Nervensystem auf Temperaturschwankungen reagieren. Mit den Greifswalder RESPONSE und ECRA Teams werde ich neurophysiologische Ableitungen von invasiven Krebsen aus verschiedenen Meeres- und Temperaturhabitaten (u.a. der Ostsee) anfertigen. Ein weiteres Ziel ist es, herauszufinden, wie genetische Variabilität zur Flexibilität des Nervensystems beiträgt. Hierzu werde ich genetische Klone einer Flusskrebsart verwenden, die sich weltweit sehr erfolgreich ausbreitet. Dieser unikale Einblick in die Konsequenzen der Meereserwärmung wird unser Verständnis physiologischer Reaktionen des Nervensystems auf wechselnde abiotische Faktoren erweitern.
Ergebnisse des Fellowships

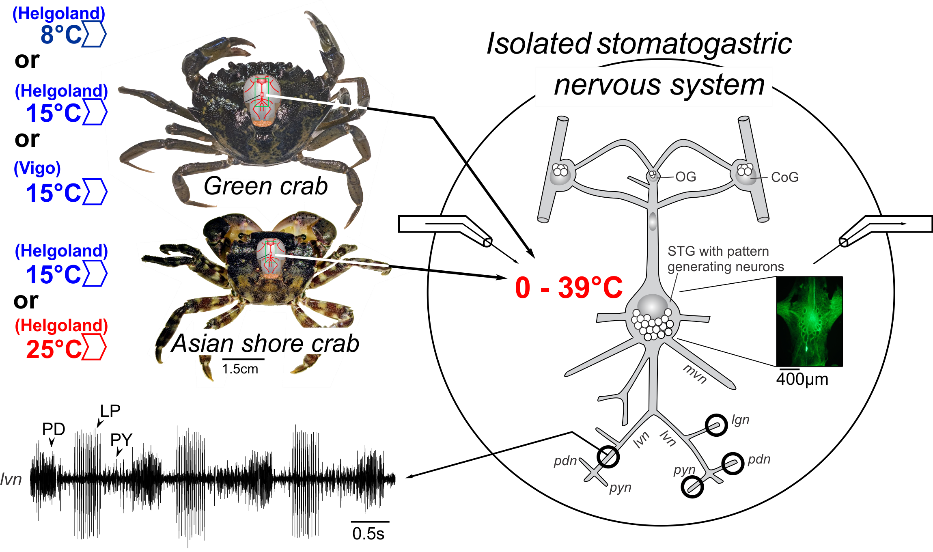
Introduction. Over the last decades, global climate change has led to dramatic changes in abiotic factors of the oceans, such as pH, salinity and oxygen, and an unprecedented increase in temperature both on average and in the total range1-4. These changes are particularly pronounced in coastal areas, where they pose a significant threat to the habitats of marine animals and dramatic shifts in animal communities have been recorded2,4-7. Decapod crustaceans, such as crabs and lobsters, are not just key species in many of the affected habitats, they are also exceptionally successful invaders. Several crab species are currently spreading around the globe, damaging and rearranging local ecosystems, with massive economic impact on the local fauna8. Much research has been invested into examining the population dynamics of these invasive species, and how environmental change facilitates their spread. There are many strategies a species or an individual from a species can adopt to cope with changing environmental conditions, and the answer to what determines survival most likely lies in the idiosyncratic abilities of each species. In the end though, any such ability must be reflected in the nervous system, because a failure of nervous system function will condemn the animal to an almost certain death. This is particularly true for crustaceans, where many vital functions are driven by neuronal ('neurogenic') activity, including heartbeat, locomotion, and feeding. An early hypothesis derived from these facts was thus that the nervous systems of invasive species must be more resilient against environmental challenges than those of others. Alternatively, invasive species may shift the range of environmental conditions in which their nervous system continues to function to fit the new conditions. Their robustness may then be unaltered or depend on other factors, such as the absolute temperature range in which the species operates. While these ideas have been around for a long time, how adaptive the nervous systems of invasive species really are has never been tested. This project tested these hypotheses by measuring the temperature robustness of neuronal activity in two invasive species of crabs.
Research questions. Three main questions were addressed: 1) How robust are nervous systems against temperature changes? 2) Do species from different habitats differ in their temperature responses? 3) Can the nervous system adapt its temperature response to new environmental temperatures?
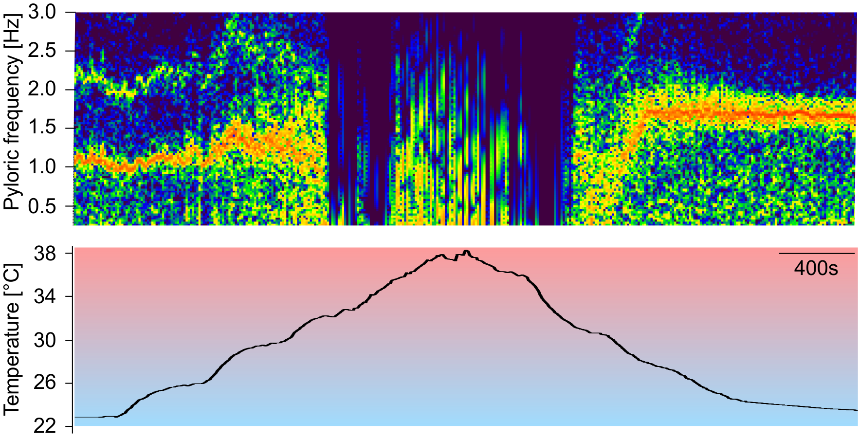
Approach. Neurophysiological recordings from green crabs, Carcinus maenas, local to Europe’s cold waters, and the Asian shore crab, Hemigrapsus takanoi, native to Japan’s waters, were used to test the range over which nervous systems produced meaningful activity (Fig, 1). Green crabs are potent invaders of the north American coast8,9. Asian shore crabs have successfully invaded the European North Sea coast in recent decades10 and appear to even replace green crabs in their native habitat. Measurements were obtained from a common-garden approach - a transplant experiment - in which nervous systems of the same species but from several different habitats were be tested for temperature robustness. Green crabs were obtained from the shores of Helgoland, a North Sea island, and from Vigo in Spain. Three groups were compared: Helgoland animals caught at 8°C, Helgoland animals caught at 8°C and acclimated to 15°C, and Vigo animals caught at 15°C. Asian shore crabs were collected in Helgoland at 15°C. A second group was collected at 15°C and then acclimated to 25°C. Electrophysiological recordings from the stomatogastric nervous system (STNS) were used. The STNS has an extensive history as a model system for studying neuronal mechanisms using neurophysiological approaches 11-15. It produces a well-characterized rhythmic activity that has been scrutinized in great detail on the cellular, circuit and modulatory levels16. Its activity is a vital component that drives digestive movements in the crustacean foregut. It is also one of the best studied neuronal microcircuits and has in the past been used to study environmental influences on circuit performance, including temperature17-23. The highly stereotyped activity pattern it generates makes it ideal for studying robustness against extrinsic perturbations. Activity patterns were recorded in the isolated STNS, while it was continuously superfused with temperature-controlled saline. Temperature was changed between 0 and 39°C. The rhythmic activity of the pyloric central pattern generator was probed using extracellular recordings.
Results. Like in other crab species, the pyloric rhythm was continuously active with a triphasic pattern and well-maintained phase relationships (N>10 for all animal groups).
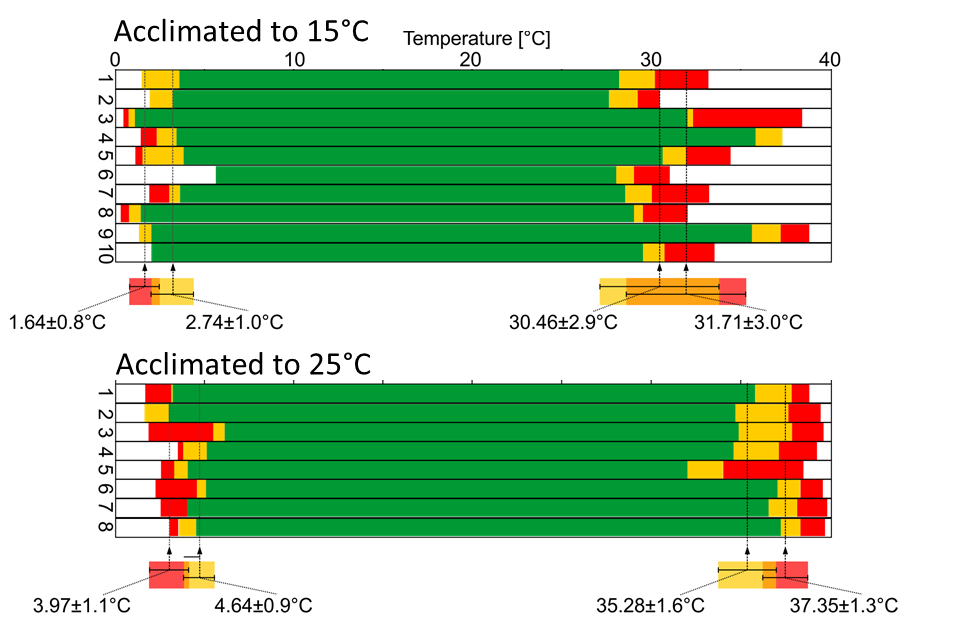
1) Neuronal activity in all tested animals was very temperature-robust. On average, the range at which neuronal activity remained stable spanned more than 30° Celsius. This was the case in both species tested. In Asian shore crabs collected from 15°C waters, first action potentials failed at 2.7±1.0°C (N=10). Rhythmic activity stopped completely at 1.6±0.8°C (N=10). At high temperature, individual action potentials or bursts failed at 30.5±2.9°C (N=10) (Fig. 2). The rhythm ultimately crashed at 31.7±3.0°C (N=10) when neurons started to fire in unpredictable ways. In green crabs collected at 8°C, first action potentials failed at 2.4±2.1°C (N=10). Rhythmic activity stopped completely at 1.2±1.4°C (N=10). At high temperature, individual action potentials or bursts failed at 30.1±1.9°C (N=10) and the rhythm crashed at 33.4±2.2°C (N=10). A return to room temperature restored rhythmic activity in all cases.
2) The overall range of temperature-robust activity was greater in green crabs. When acclimated to the same temperature (15°C), green crabs were more heat-resistant than Asian shore crabs. In 15°C acclimated green crabs, first action potentials failed at 3.7±0.5°C (N=9). Rhythmic activity stopped at 1.8±0.5°C (N=9). This was not significantly different from Asian shore crabs (2.7±1.0°C, and 1.6±0.8°C, respectively; N=10). At high temperature, action potentials or bursts started to fail at 33.3±0.9°C (N=9) and the rhythm crashed at 37.4±1.1°C (N=9). This was significantly higher than Asian shore crabs (30.5±2.9°C, and 31.7±3.0°C, respectively; N=10). One-way-Anova with SNK posthoc test at P<0.05 was used in all statistical analyses.
3) Exposing animals to higher habitat temperatures for several weeks moved and expanded the temperature range in which robust activity was generated. In both species the upper crash temperature increased slow through homeostatic processes. When acclimated from 15°C to 25°C (Fig. 3), the crash of rhythmic activity in Asian shore crabs at cold temperatures increased significantly from 1.6±0.8°C (N=10) to 4.0±1.1°C (N=8). The upper crash temperature increased significantly from 31.7±3.0°C (N=10) to 37.4±1.3°C (N=8). In green crabs, when acclimated from 8°C to 15°C, the cold crash temperature remained the same (1.2±1.4°C and 1.8±0.5°C, respectively; N=10). The upper crash temperature increased significantly from 33.4±2.2°C (N=9) to 37.4±1.1°C (N=9). Crabs from Helgoland and acclimated to 15°C were not significantly different from crabs collected in Spain at 15°C. One-way-Anova with SNK posthoc test at P<0.05 was used in all statistical analyses.
In summary, the crustacean nervous system shows a remarkable robustness against temperature changes, and it can adjust its temperature response to the habitat temperature. Differences between species that live in the same ecological niche may contribute to being a successful invader of new abitats.
Impact: The main driver of global climate change is temperature. Maintaining neural function at different temperatures is a particularly difficult challenge for the nervous system since all physiological processes, including those underlying communication and excitability in the brain, are temperature-dependent24. Aquatic invertebrates are particularly susceptible to such influences, because they do not actively control their body temperature, and need to continuously flood their gills with the (over-)heated and less oxygenated water. Adequate functioning of the nervous system is crucial for development (e.g. neurohormonal release), survival (e.g. heartbeat) and reproduction (e.g. mate selection) of these animals. This project filles a gap in our knowledge between the ecological effects of climate change, and the potential physiological mechanisms that drive the ecological responses. The results demonstrate the extreme temperature robustness of the investigated invasive crab species, and their ability to adjust to new temperature habitats.
Innovation: This project embarked on the currently understudied field of neuro-eco-physiology. While much is known about the ecology of many animal species, and about the effects that changes in environmental conditions have on their behavior and survival, our understanding of the physiological mechanisms that drive behavioral changes and adaptations is still in its infancy. The project had several innovate components: 1) it addressed the gap in our understanding how global climate change affects animals and their behaviors through studying the effects of temperature on the nervous system. 2) Few, if any, studies have focused on how the nervous systems of species inhabiting similar temperature habitats compare in their response to environmental change. Studying invasive species, champion animals when it comes to global climate change, to ask how their nervous systems adjusts to different habitats and environmental challenges is also a new approach. 3) We used a common-garden approach, which allowed us to work the Neuro-eco-physiology axis that is mostly absent, but direly needed, in today's research.
Outcomes: The project led to one poster presentation, two seminar presentation, and two invitations:
- Poster: Stein, W, & Harzsch, S. (2022). Temperature responses of stomatogastric neurons in the brush-clawed shore crab, Hemigrapsus takanoi. International Society for Neuroethology, Lisbon, Portugal.
- Talk 1: Stein, W, & Harzsch, S. (2022). Temperature resilience of neuronal activity patterns in the brush-clawed shore crab, Hemigrapsus takanoi. German Zoological Society Annual Meeting, Bonn, Germany.
- Talk 2: Stein, W (2022). Some like it hot: invasive brains in hot waters. Monthly dynamic neural network meeting. Scheduled for October 26, 2022.
- Invitation 1: Neurobiology and Changing Ecosystems. Workshop, Kavli Foundation, Los Angeles. Scheduled for November 16 - 28, 2022.
- Invitation 2: Invited seminar for the Symposium "How cellular clocks spanning multiple time scales orchestrate biological timing", 15th Göttingen Meeting of the German Neuroscience Society. Scheduled for March 22, 2023. Session organizers Monika Stengl & Martin Garcia (Kassel, Germany).
In addition, the generated data are currently being analyzed for publication. We are aiming for two concurrent manuscripts in the Journal of Experimental Biology (The company of Biologists).
Further outcomes and achievements. The fellowship also provided me with the time to finalize data analyses of several related projects, and to submit and publish 5 manuscripts in peer-reviewed journals.
- Stein, W, et al. (2022) Frontiers in Physiology, 13:947598; doi: 10.3389/fphys.2022.947598.
- Städele, C., Stein, W. (2022) . Frontiers in Cellular Neuroscience, 16: 849160. doi: doi.org/10.1101/2021.11.04.467352
- Stein, W. Harris, A.L. (2022) Journal of Computational Neuroscience, 50, 275–298. doi: doi.org/10.1101/2021.04.25.441350
- Stein, W., et al. (2022) Journal of Neurophysiology. doi: 10.1152/jn.00545.2021. PMID: 35171723.
- DeMaegd, M. L., Stein, W. (2021). Journal of Neuroscience. 41.36: 7607-7622.
A further manuscript is in its final stage and will be part of a handbook for the study of the enteric nervous system: Stein, W. (2023) First brains. Invertebrate models of enteric nervous system. In: Primer Enteric Nervous System (Springer). Scheduled for submission in October 2022.
- I gave a lecture at the University of Greifswald entitled "Grundlagen der zellulären Neurophysiologie in Arthropoden: Von Ionenkanälen zu neuronalen Netzwerken." im Rahmen der Sondervertiefungsrichtung Neurobiologie BSc. Humanbiologie, Übung „Methoden in den Neurowissenschaften“.
- I participated in the "Zeit" workshop at the Krupp Kolleg, with a primer entitled "Wie Hirnzellen Zeit machen".
- I was a regular participant of the weekly seminars of Dr. Harzsch's group, Dr. Uhl's group, and of the Arthropod Neuroscience Network.
- Finally, the fellowship allowed me to strengthen my collaborations with two German researchers: Dr. Frank Lyko (DKFZ; project on crustacean brain cell development) and Dr. Carola Städele (Univ Gottingen; project on tick neurobiology).
References for project report.
1 Boersma, M. et al. Projecting effects of climate change on marine systems: is the mean all that matters? Proc. Biol. Sci.283, doi:10.1098/rspb.2015.2274 (2016).
2 Burrows, M. T. et al. The pace of shifting climate in marine and terrestrial ecosystems. Science334, 652-655, doi:10.1126/science.1210288 (2011).
3 Reid, P. C. et al. Chapter 1. Impacts of the oceans on climate change. Adv Mar Biol56, 1-150, doi:10.1016/S0065-2881(09)56001-4 (2009).
4 Molinos, J. G. et al. Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Change.6, 83 (2016).
5 Beaugrand, G., Luczak, C. & M, E. Rapid biogeographical plankton shifts in the North Atlantic Ocean. Global Change Biology15, 1790-1803, doi:10.1111/j.1365-2486.2009.01848.x (2009).
6 Poloczanska, E. S. et al. Global imprint of climate change on marine life. Nat. Clim. Change.3, 919 (2013).
7 Boyd, P. W. et al. Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change-A review. Glob Chang Biol24, 2239-2261, doi:10.1111/gcb.14102 (2018).
8 Leignel, V., Stillman, J. H., Baringou, S., Thabet, R. & Metais, I. Overview on the European green crab Carcinus spp. (Portunidae, Decapoda), one of the most famous marine invaders and ecotoxicological models. Environ Sci Pollut Res Int21, 9129-9144, doi:10.1007/s11356-014-2979-4 (2014).
9 Young, A. M. & Elliott, J. A. Life history and population dynamics of green crabs (Carcinus maenas). Fishes5, 4 (2020).
10 Gollasch, S. The asian decapodHemigrapsus penicillatus (de Haan, 1835)(Grapsidae, Decapoda) introduced in European waters: status quo and future perspective. Helgoländer Meeresuntersuchungen52, 359-366 (1998).
11 Temporal, S., Lett, K. M. & Schulz, D. J. Activity-dependent feedback regulates correlated ion channel mRNA levels in single identified motor neurons. Curr Biol24, 1899-1904, doi:10.1016/j.cub.2014.06.067 (2014).
12 Temporal, S. et al. Neuromodulation independently determines correlated channel expression and conductance levels in motor neurons of the stomatogastric ganglion. J. Neurophysiol.107, 718-727 (2012).
13 Schulz, D. J., Goaillard, J. M. & Marder, E. E. Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc. Natl. Acad. Sci. U. S. A.104, 13187-13191 (2007).
14 Schulz, D. J., Goaillard, J. M. & Marder, E. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat. Neurosci.9, 356-362 (2006).
15 Khorkova, O. & Golowasch, J. Neuromodulators, not activity, control coordinated expression of ionic currents. J. Neurosci.27, 8709-8718 (2007).
16 Stein, W. in Oxford Research Encyclopedia, dx.doi.org/10.1093/acrefore/9780190264086.013.153 (Oxford University Press, 2017).
17 Bucher, D., Haspel, G., Golowasch, J. & Nadim, F. Central pattern generators. eLS (2009).
18 Nadim, F. & Bucher, D. Neuromodulation of neurons and synapses. Curr. Opin. Neurobiol.29C, 48-56, doi:10.1016/j.conb.2014.05.003 (2014).
19 Svensson, E. et al. General Principles of Neuronal Co-transmission: Insights From Multiple Model Systems. Front. Neural Circuits12, 117 (2019).
20 Nusbaum, M. P. in Encyclopedia of Neuroscience Encyclopedia of Neuroscience (ed M.D. Binder, Hirokawa, N., Windhorst, U.) (Springer, Berlin, Heidelberg, 2013).
21 Nusbaum, M. P. & Blitz, D. M. Neuropeptide modulation of microcircuits. Curr. Opin. Neurobiol.22, 592-601, doi:10.1016/j.conb.2012.01.003 (2012).
22 Nusbaum, M. P. & Beenhakker, M. P. A small-systems approach to motor pattern generation. Nature417, 343-350 (2002).
23 Stein, W. Modulation of stomatogastric rhythms. J. Comp. Physiol. A195, 989-1009 (2009).
24 Robertson, R. M. & Money, T. G. Temperature and neuronal circuit function: compensation, tuning and tolerance. Curr. Opin. Neurobiol.22, 724-734, doi:10.1016/j.conb.2012.01.008S0959-4388(12)00010-4 [pii] (2012).
